Fire Sources
Defining Fire
Fire can be described in many ways - here are a few:
NFPA 921: "A rapid oxidation process, which is a chemical reaction resulting in the evolution of light and heat in varying intensities."
Webster's Dictionary: "A fire is an exothermic chemical reaction that emits heat and light" Fire can also be explained in terms of the Fire Tetrahedron - a geometric representation of what is required for fire to exist, namely, fuel, an oxidizing agent, heat, and an uninhibited chemical reaction.
Combustion
Combustion is a chemical reaction which basically involves three substances: fuel, oxygen and combustion products. It is a fast oxidation process, the creation of combustion products out of oxygen and fuel, typically based on exothermic processes, but not exclusively.
In order to have a sustained fire, multiple criteria must be satisfied. They are illustrated by the fire tetrahedron as indicated in the following figure. Thus a fire needs, the fuel, an oxydant, here oxygen, which reacts with the fuel and converts chemically bound energy into heat, a supply of heat, which is needed to overcome the activation energy of the chemical reaction, and sufficiently high reaction rates to keep up a chain reaction.
Fire Triangle

Measuring Fire
Heat Energy is a form of energy characterized by vibration of molecules and capable of initiating and supporting chemical changes and changes of state (NFPA 921). In other words, it is the energy needed to change the temperature of an object - add heat, temperature increases; remove heat, temperature decreases. Heat energy is measured in units of Joules (J), however it can also be measured in Calories (1 Calorie = 4.184 J) and BTU's (1 BTU = 1055 J).
Temperature is a measure of the degree of molecular activity of a material compared to a reference point. Temperature is measured in degrees Farenheit (melting point of ice = 32 º F, boiling point of water = 212 º F) or degrees Celsius (melting point of ice = 0 º C, boiling point of water = 100 º C).
Fire Sources Creation in FRI3D
Based on the concepts above, FRI3D's user interface has been designed so as to be able for the user to enter those values to be picked up by the simulation.
Fire Sources are created in FRI3D from the Compartment Inspector by
Simulation Items -> New Fire Source
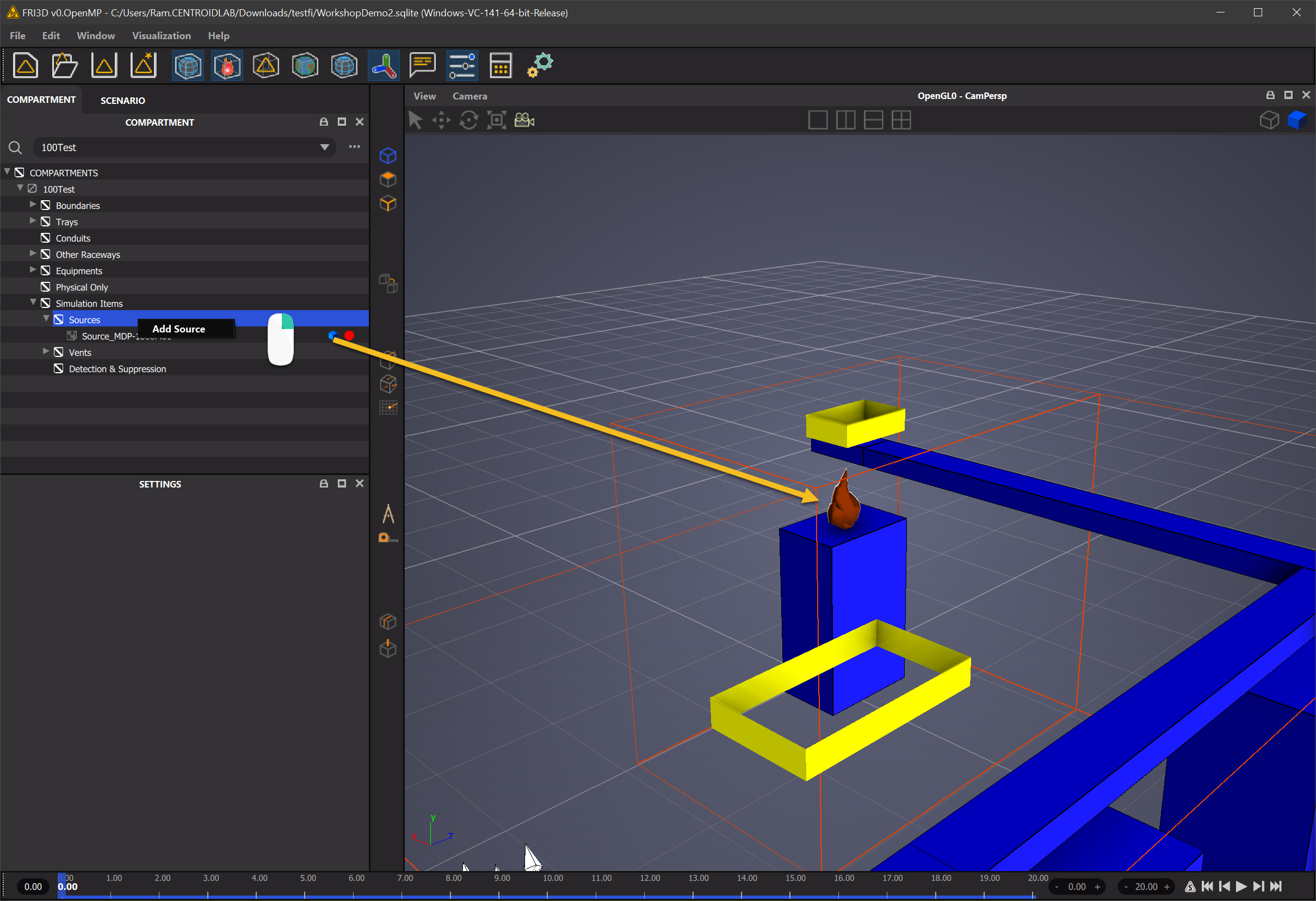
Fire Properties in FRI3D
FRI3D has fire sources with an assigned fire material. The fire source has the spatial information and the fire material defines properties such as the heat release rate as shown. A fire material can be assigned to multiple fire sources. These properties are used to assign fires and fire material specifications for the simulation (FDS/CFAST or other)
The fire source properties are created and edited by
Fire Source -> Properties & Reactions
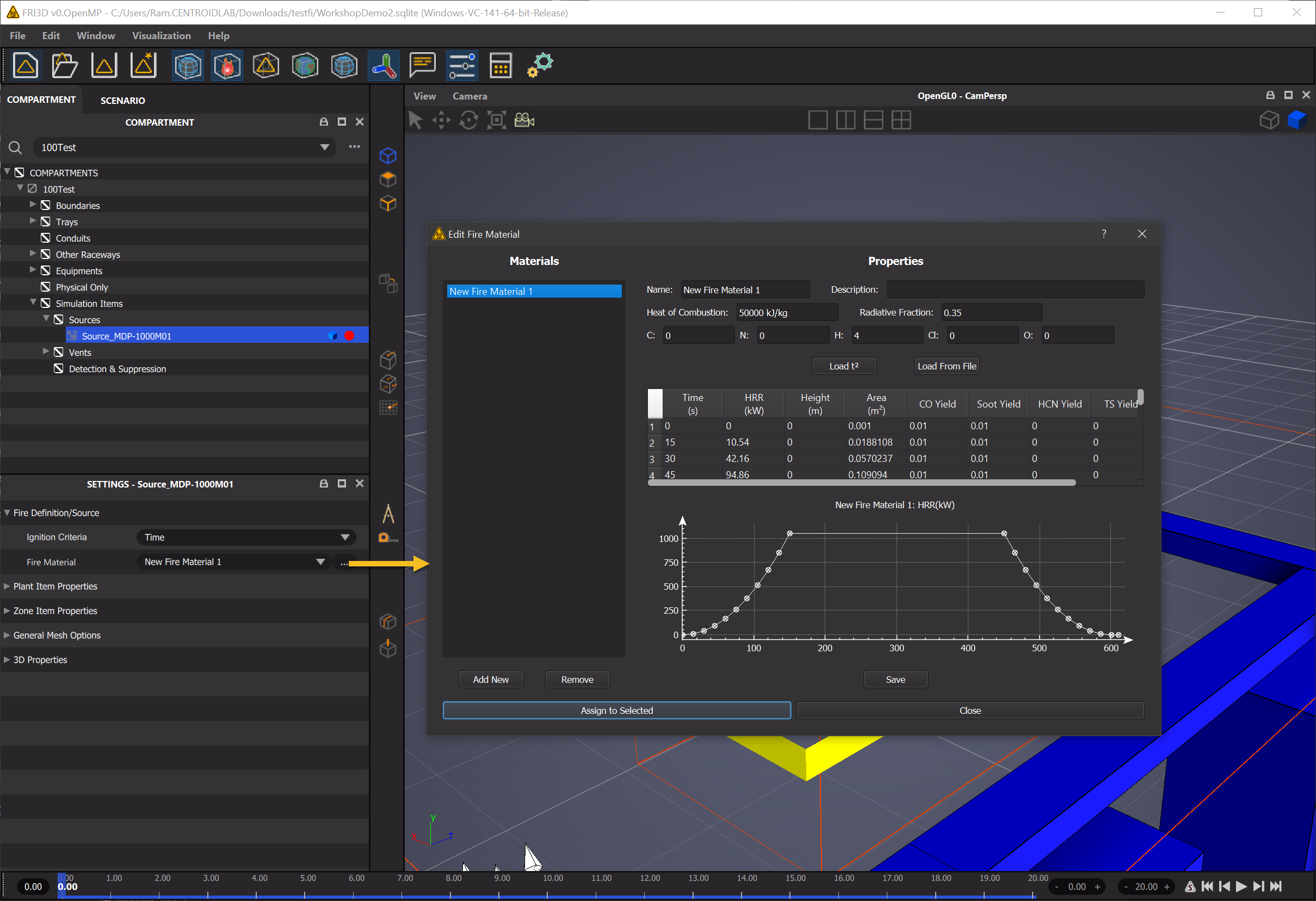
The following sections list the properties of fire as modeled by FRI3D. The specifics of each term however is dependent on the exact simulation system used (ie: CFAST/FDS etc). The details pertaining to simulation specific are in subsequent sections.
There are two kinds of fires currently supported by FRI3D. Solid and Liquid fire sources. For the case of liquid fires some of the needed components for the specific fire simulator (CFAST or FDS) are calculated. Some parameters are common among both. For Example Heat of Combustion etc.
Prebuilt Fire Source Library
FRI3D packages a library of hundreds of fire sources comes with the software and is ready to be used for the most common fires occuring in a plant. They can be upgrated anytime according to the latest guidelines for such fire scenarios. The FRI3D fire library catagorizes the fires based on solid fuels (Batteries etc) and liquid fuels (Benzene etc)
Heat of Combusion
During combustion, not only chemical bounds are rearranged, but heat is released. This is quantified as heat of combustion.
This is the energy released as heat when a compound undergoes complete combustion with oxygen under standard conditions. The chemical reaction is typically a hydrocarbon reacting with oxygen to form carbon dioxide, water and heat.
This number is specified in KJ/kg and is the amount of energy released per unit mass of fuel consumed. The database of fires prescribes this value depending on the type of fire.
Radiative Fraction
The most important radiation parameter is the fraction of energy released from the fire as thermal radiation, commonly referred to as the radiative fraction. The Radiative fraction specified as part of the fire properties.
By default, the radiative fraction is 35%, meaning that in mesh cells with combustion, 35% of the Heat Release Rate per Unit Volume (HRRPUV) will be released as thermal radiation.
Reaction (Stoichiometry C, N, H, Cl, O) and Volume Fractions
Most Fuels are mainly compounds of carbon and hydrogen. The reaction of its oxidation can by written by the equation of stoichiometry.
Stoichiometric or Theoretical Combustion is the ideal combustion process where fuel is burned completely.
A complete combustion is a process burning all the carbon (C) to (CO 2 ), all the hydrogen (H) to (H 2 O) and all the sulphur (S) to (SO 2 ).
With unburned components in the exhaust gas such as C, H 2 , CO, the combustion process is uncompleted and not stoichiometric .
The combustion process can be expressed:
[C + H (fuel)] + [O 2 + N 2 (Air)] -> (Combustion Process) -> [CO 2 + H 2 O + N 2 (Heat)]
where
C = Carbon H = Hydrogen O = Oxygen N = Nitrogen
The Stoichiometric coefficients representing these components are specified in the fire properties.
The most common oxidizer is air . The chemical equation for stoichiometric combustion of methane - CH 4 - with air can be expressed as
CH 4 + 2(O 2 + 3.76 N 2 ) -> CO 2 + 2 H 2 O + 7.52 N 2
If more air is supplied some of the air will not be involved in the reaction. The additional air is termed excess air , but the term theoretical air may also be used. 200% theoretical air is 100% excess air.
The chemical equation for methane burned with 25% excess air can be expressed as
CH 4 + 1.25 x 2(O 2 + 3.76 N 2 ) -> CO 2 + 2 H 2 O + 0.5 O 2 + 9.4 N 2
The fire material library specification has these ratios present as well.
Solid Fires
Heat Release Rate
Heat Release Rate (HRR) is the rate at which fire releases energy - this is also known as power. HRR is measured in units of Watts (W), which is an International System unit equal to one Joule per second. Depending on the size of the fire, HRR is also measured in Kilowatts (equal to 1,000 Watts) or Megawatts (equal 1,000,000 Watts).
Typically, the heat release rate (heat energy evolving on a per unit time basis) of a fire 0 (kW) changes as the size of the fire changes, as a function of time t (seconds) after fire ignition.
There are four stages of fire development: ignition, growth, fully developed, and decay. The ignition stage is when all four elements of the fire tetrahedron coalesce, the fuels reach their ignition temperature, and the fire begins.
Soot Yield
This is the fraction of soot from the fuel. The fraction of fuel mass converted into smoke particulate. This is expressed as a unitless number (kg/kg) and by default 0.01.
CO Yield
This the fraction of fuel mass converted into carbon monoxide(kg/kg) and by default is 0. This is also specified as the fire property.
Height and Area
These properties in subsequent versions would be calculated from the size of the fire source. Currently however this number can be expressed as part of the fire property.
Liquid Fires
Seconday Fire Sources
Fire Material Library in FRI3D
For detailed fire modeling, each item identified as a potential fire source under the fire analysis needs to have relevant data such as ignition frequency and a heat release rate (HRR). The fire simulation code uses the HRR for the fire growth and to perform the simulation analysis. The information is present in FRI3D as different types of fire source types for easy selection and use. When the user creates a new fire source, they assign a specific fire material from a list.
From the fire protection reports from the Nuclear Regulatory Comiision and Society of Fire Protection Engineers (SFPE) Handbook the data is collected and organized into two tabs one for solid fire materials, one for liquid fire materials.
Fire Source -> Properties & Reactions
